【How SHNU research is transforming our lives】
Using Sunlight to Recover Precious Metals

Date: March 25, 2021
Source: College of Chemistry and Materials Science, Shanghai Normal University
SHNU Researchers, led by Prof. Hexing Li and Prof. Zhenfeng Bian from the SHNU-based Key Laboratory of Resource Chemistry of the Ministry of Education, report today that they have discovered a universal and environmentally friendly method to recover precious metals using photocatalytic technology. No need for strong acids, strong bases and strong corrosive solvents, or even electrochemical assistance, this new method can realize 100% photocatalytic oxidation dissolution of various precious metals (gold, silver, palladium, platinum, rhodium, ruthenium and iridium, etc.) by using just sunlight and photocatalysts such as titanium dioxide (TiO2). So far, it is the most advanced, cleanest, most convenient and cheapest precious metal recycling technology in the world. This is a breakthrough change in precious metal smelting, and it is also a milestone in the development and application of photocatalysis theory.
Note: This result was recently published online in Nature Sustainability, Selective recovery of precious metals through photocatalysis” (https://doi.org/10.1038/s41893-021-00697-4). Ms. Yao Chen is the first author of the paper, andProf. Zhonglin Wang from Beijing Institute of Nano Energy and Systems, Chinese Academy of Sciences, Prof. Hexing Li and Prof. Zhenfeng Bian from College of Chemistry and Materials Science, Shanghai Normal University co-corresponded the paper. The authors have filed a patent application (US Patent application no. 17042775) on technology related to the processes described in this Article.

Full story:
Harnessing Sunlight to Recover Precious Metals
Precious metals mainly refer to gold, silver and platinum group metal elements, which have good physical properties, high chemical stability and strong corrosion resistance. They are widely used in electronic equipment, chemical catalysts, alloy devices, etc. However, precious metal resources are limited and expensive. Therefore, recycling precious metals is an important industry with a huge market at home and abroad.
At present, the methods for industrial recovery of precious metals mainly include pyrometallurgy and hydrometallurgy. Pyrometallurgy takes a long time and consumes a lot of energy. Hydrometallurgy involves the use of corrosive and toxic cyanide, aqua regia and thiourea, causing serious damage to the environment. It is necessary to develop an environmentally friendly, low-cost and efficient new method to dissolve precious metals.
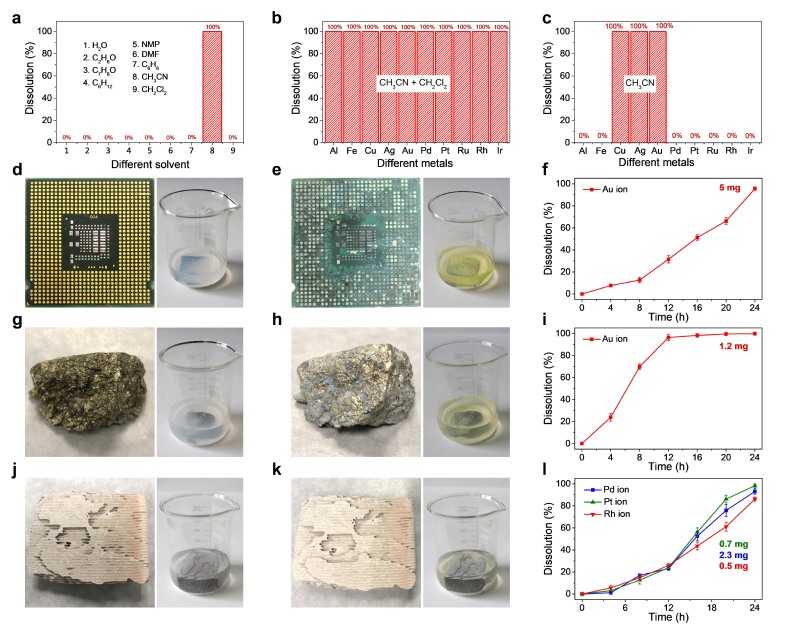
Team led by Prof. Hexing Li and Prof. Zhenfeng Bian have long been engaged in photocatalytic technology for environmental pollution control. Under mild conditions, photocatalysis can generate highly reactive free radicals, which can be used for pollutant degradation, sterilization, hydrogen production and heavy metal ion reduction. It has the advantages of simple operation, low energy consumption and no secondary pollution.
For the first time, the team developed a universal and environmentally friendly method for dissolving precious metals by using photocatalytic technology. No need for strong acids, strong bases and strong corrosive solvents, or even electrochemical assistance. It can realize 100% photocatalytic oxidation dissolution of various precious metals (gold, silver, palladium, platinum, rhodium, ruthenium and iridium, etc.). In addition, the catalyst and solvent can be reused, reducing environmental pollution effectively. The selective dissolution of precious metals can also be achieved, which simplifies the separation process in the recovery of precious metals. The method is also suitable for electronic waste, automobile three-way catalysts, industrial catalysts and ore. So far, it is the most advanced, cleanest, most convenient and cheapest precious metal recycling technology in the world. This is a breakthrough change in precious metal smelting, and it is also a milestone in the development and application of photocatalysis theory.

